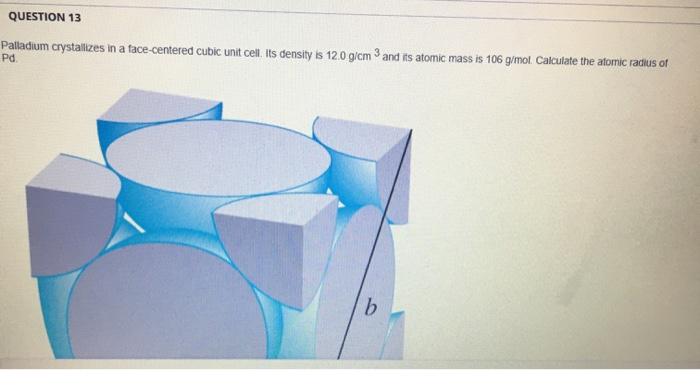
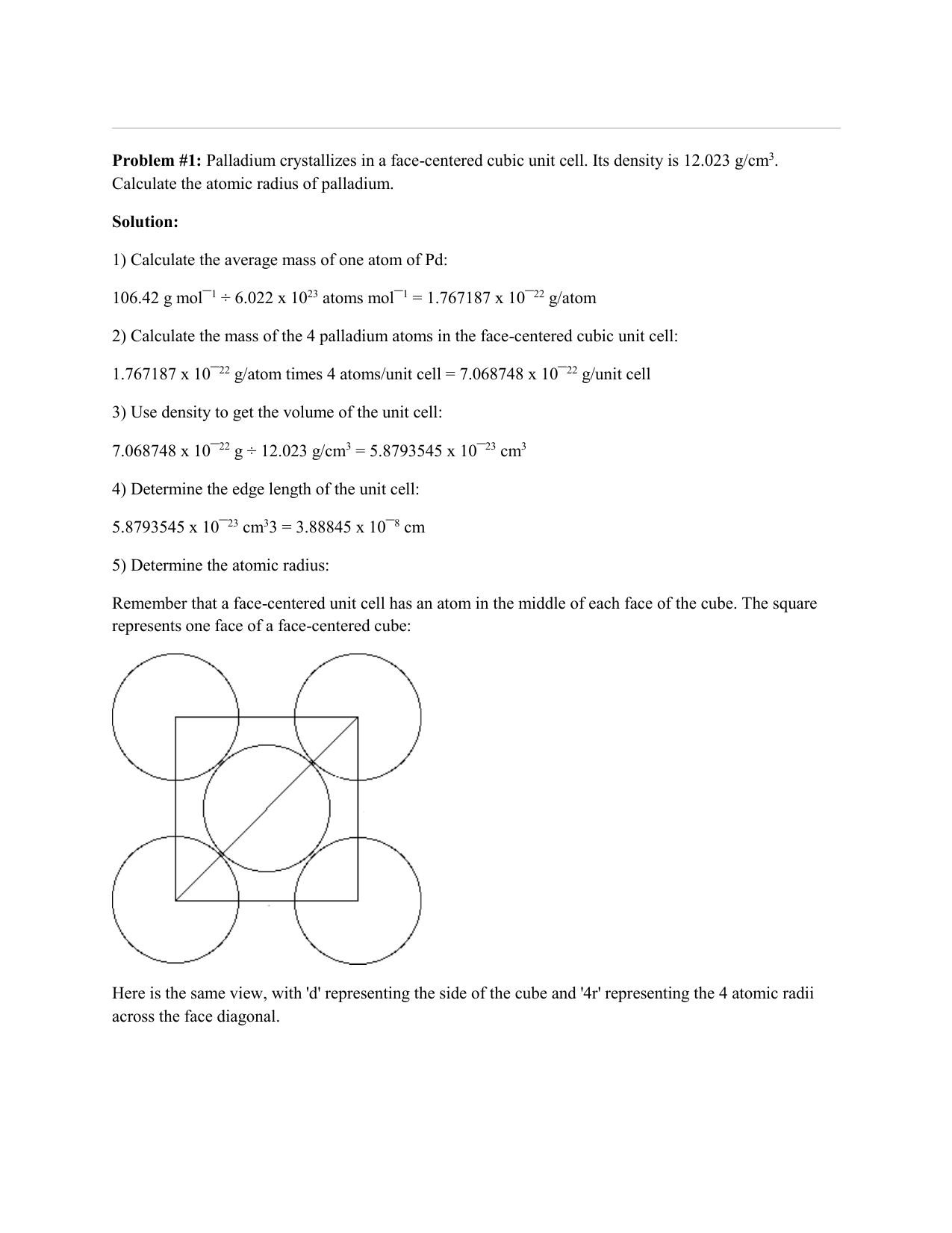
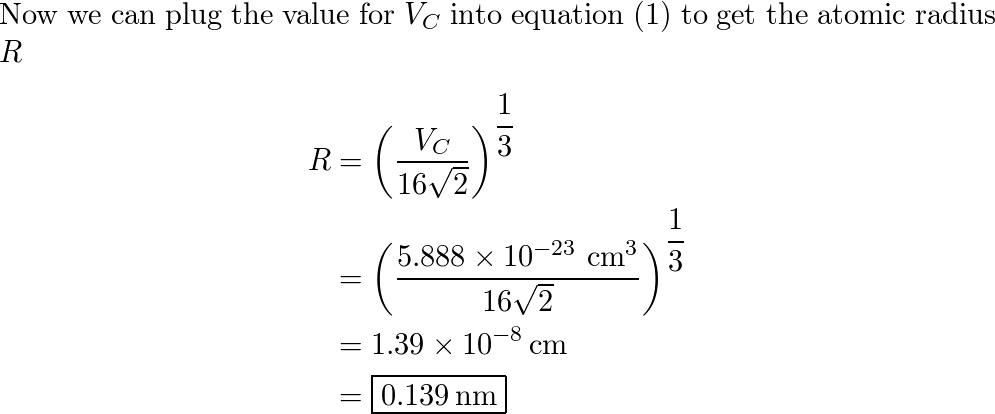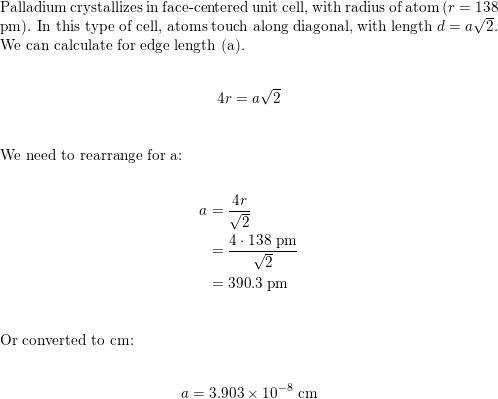Document - Problem #1: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium. | Course Hero

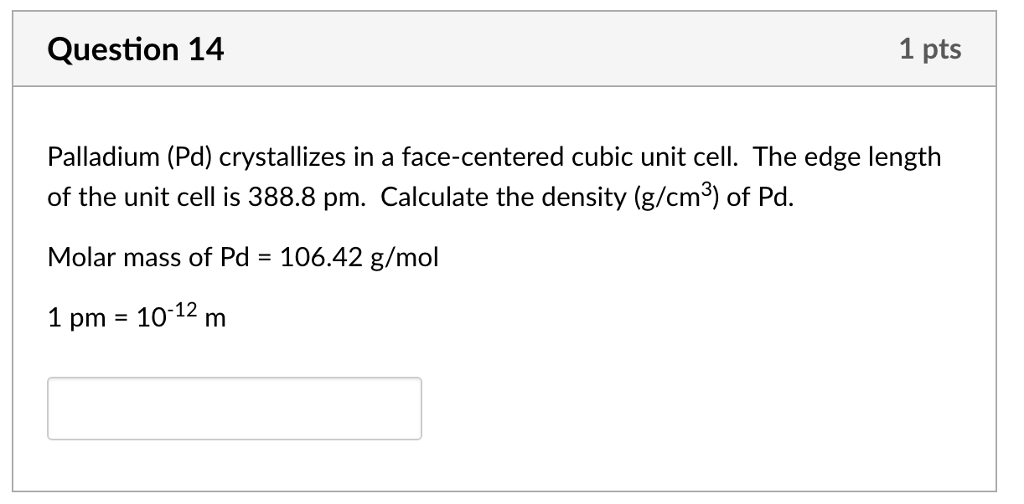
The metal palladium crystallizes in a face-centered cubic lattice with an edge length of 388.8 pm. What is the density of the palladium? | Homework.Study.com
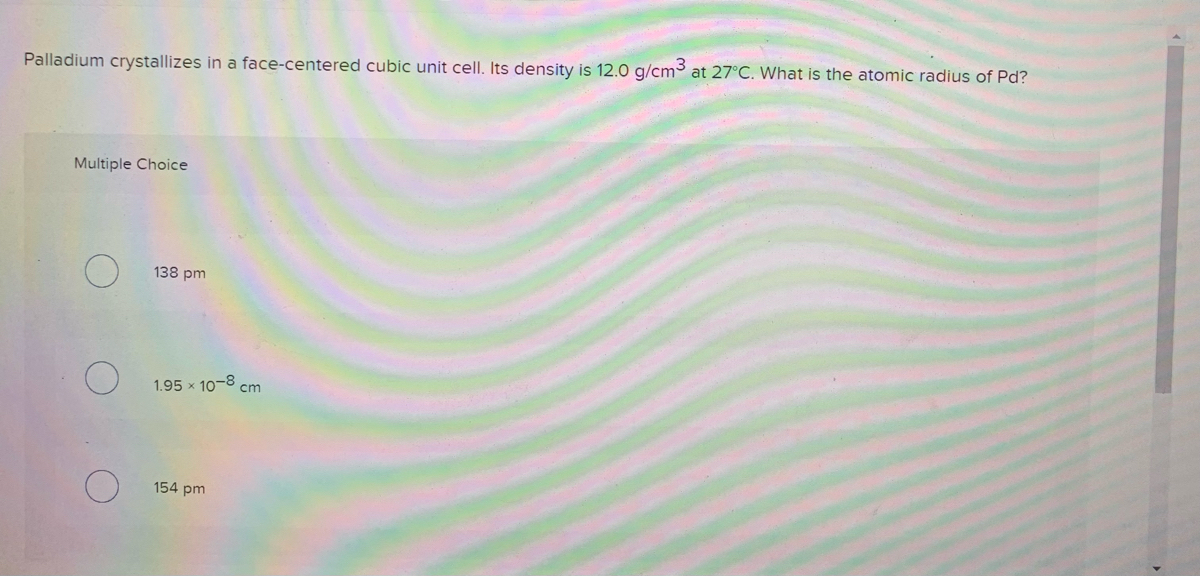
SOLVED: Palladium crystalllzes In face-centered cubic unit cell. Its density i5 12.0 g/cm 27"€. Calculate the atomic radius of palladium 138 pm (b) 1.95 * 10 " nm (c) 1.95 * 10 cm (d) 154 pmn (e) 0.109 nm

Gold occurs as face centred cube and it has a density of 19.30 kg dm ^-3 .Calculate atomic radius of gold. (Molar mass of Au = 197 )
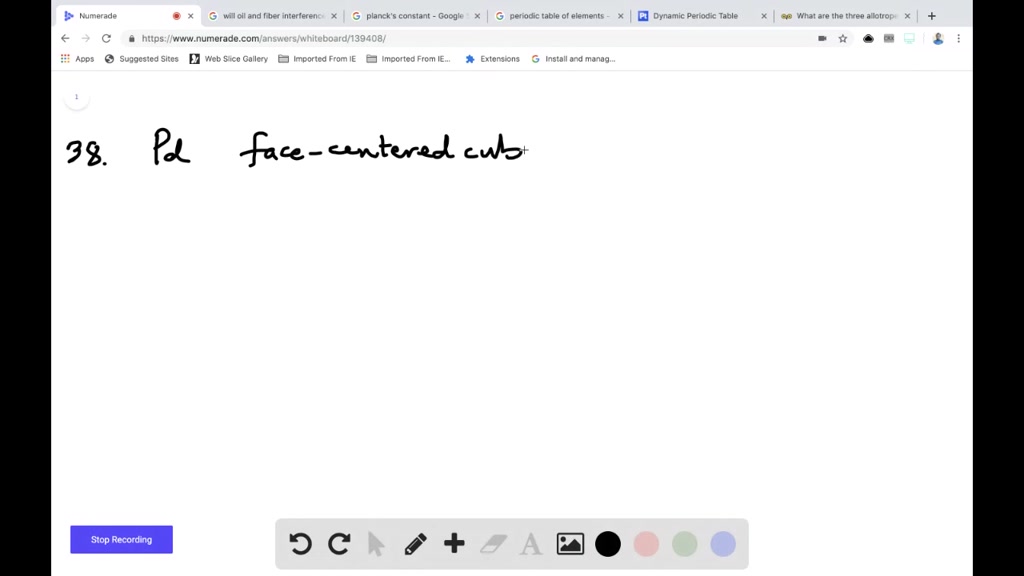
SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g / cm^3, a radius of 138 pm, and a molar mass of 106.42 g / mol . Use these data to calculate Avogadro's number.
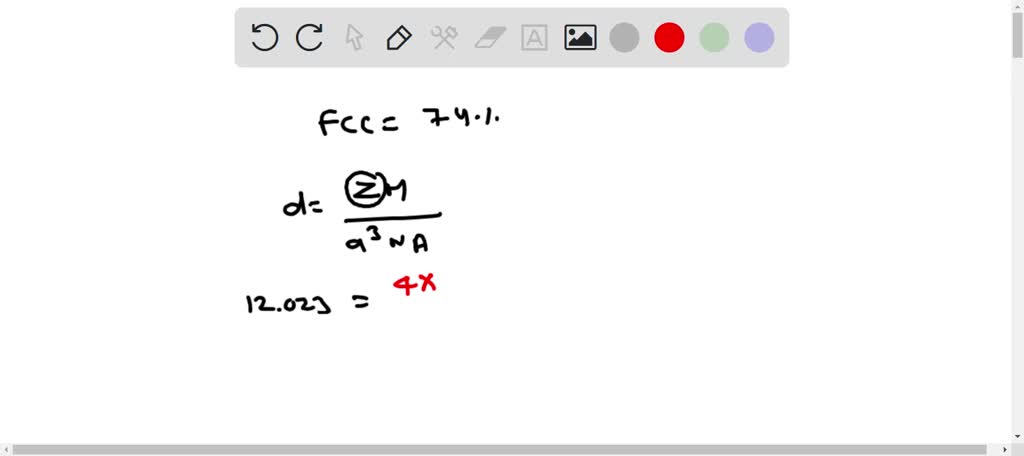
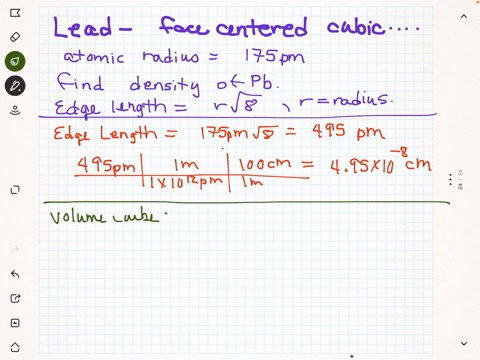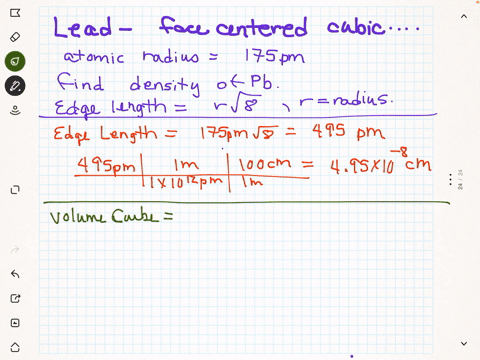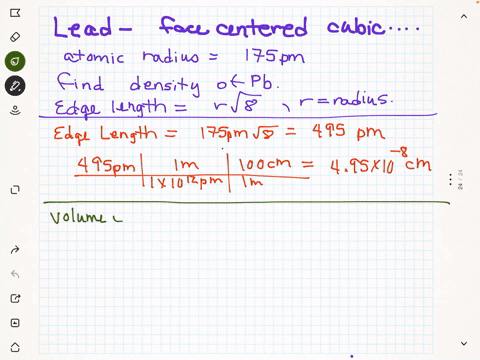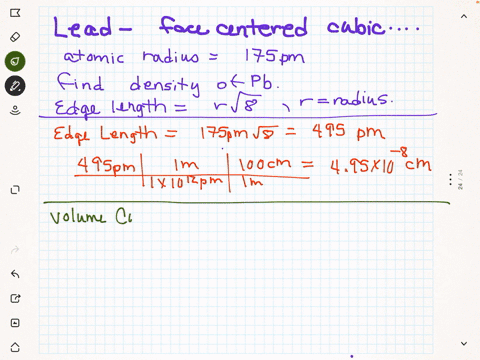
SOLVED: Palladium crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3. Calculate the atomic radius of palladium.

SOLVED:Palladium crystallizes with a face-centered cubic structure. It has a density of 12.0 g/cm3, a radius of 138 pm, and a molar mass of 106.42 g/mol. Use these data to calculate Avogadro's

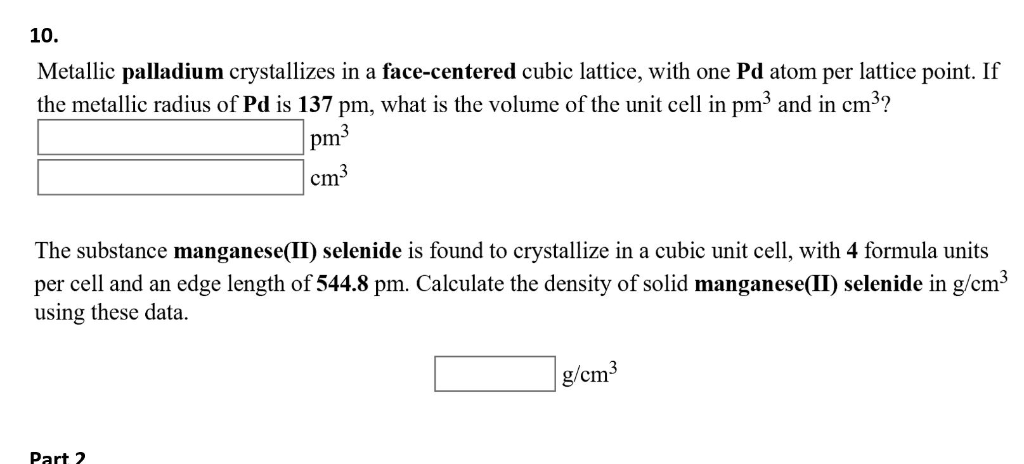
SOLVED: Metallic palladium crystallizes in a face-centered cubic lattice, with one Pd atom per lattice point. If the metallic radius of Pd is 137 pm, what is the volume of the unit